In a groundbreaking study recently published in Cell Host & Microbe, researchers from the Georgia State University Institute for Biomedical Sciences have unveiled an important connection between the composition of gut microbiota and the severity of respiratory viral infections (RVIs). The paper, titled “Intestinal Microbiota Programming of Alveolar Macrophages Influences Severity of Respiratory Viral Infection,” provides compelling insights into the role of segmented filamentous bacteria (SFBs) in fortifying mice against a range of respiratory viruses, including influenza, respiratory syncytial virus (RSV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Interplay between gut microbiota and immune response to respiratory viruses
The study, co-authored by Andrew Gewirtz, PhD, Regents’ Professor at the Institute for Biomedical Sciences, and Richard Plemper, PhD, Regents’ Professor and director of the Center for Translational Antiviral Research, sheds light on the intricate interplay between gut microbiota and the immune response to respiratory viruses.
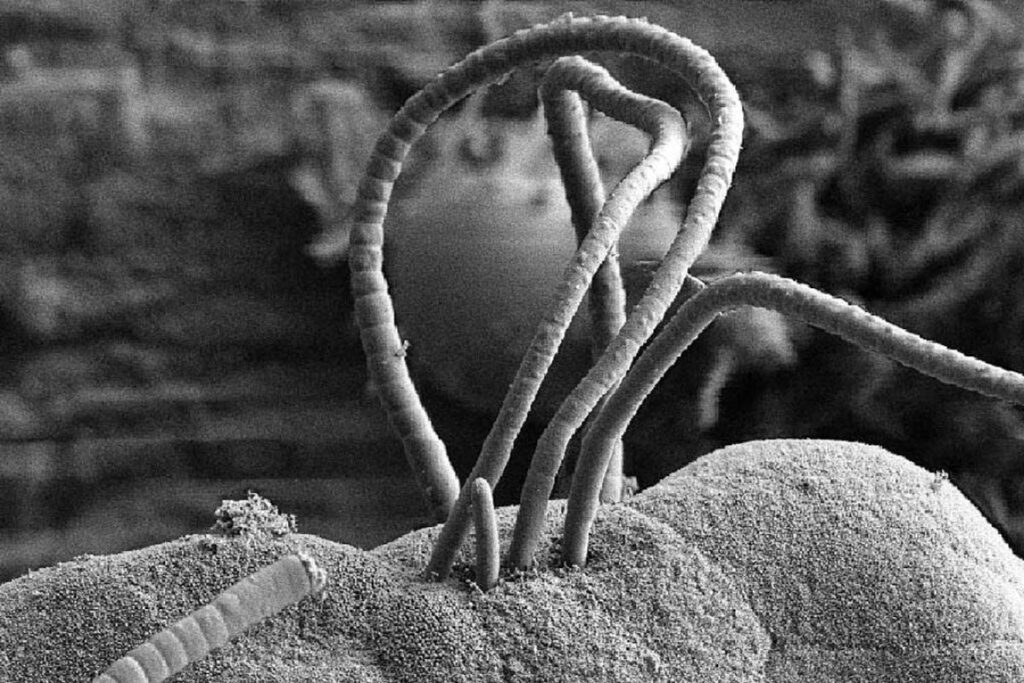
The research focused on mice with distinct microbiome variations, investigating the impact of gut microbiota on susceptibility and severity of respiratory virus infections. Particularly, it was observed that the presence or absence of SFBs played an important role in shaping the outcomes of viral infections. Whether naturally acquired or administered, SFBs exhibited a remarkable protective effect against influenza virus. The same effect was also detected against RSV and SARS-CoV-2.
The authors emphasized the significance of gut microbiota composition, stating, “We hypothesized that one important factor is gut microbiota composition, which is now appreciated to have broad influence over a range of chronic inflammatory diseases and the immune responses with which such diseases are associated.”
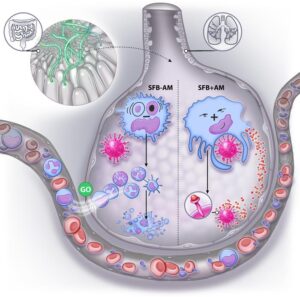
Modulation of basally resident alveolar macrophages
One of the pivotal findings of the study lies in the modulation of basally resident alveolar macrophages, the immune cells located in the lungs that act as the first responders to inhaled threats, including respiratory viruses. In mice lacking SFBs, depletion of these alveolar macrophages rapidly occurred with the progression of the influenza virus A infection. However, in SFB-colonized mice, these immune cells were somehow altered to acquire resistance against influenza virus-induced depletion and inflammatory signaling.
The researchers noted, “We show herein that how AMs respond to respiratory viruses is dramatically altered by the intestinal microbiota composition. Specifically, we report that colonization of the intestine by SFB, naturally acquired or exogenously administered, markedly attenuates influenza A viruses (IAV)-induced AM pro-inflammatory gene expression while increasing the ability of AMs to disable IAV.”
Importance of Complement System
Moreover, the study highlighted the pivotal role of the complement system, a component of the immune system, in the defense against the influenza virus. In fact, basally resident alveolar macrophages, stimulated by SFB, disabled the virus by activating the complement system, thus providing a layer of protection against severe respiratory infections.
The collective results indicate that for SFBs to exert their protective influence, the presence of basally resident alveolar macrophages in the lungs is essential. This revelation holds significant implications for understanding the dynamics of respiratory infections in humans, potentially offering a new avenue for risk assessment regarding the progression to severe disease following infections.
Future prospective
In concluding their study, the authors offered a forward-looking perspective, stating, “It is improbable that SFB is the only gut microbe capable of impacting AMs and susceptibility to RVI. Rather, we posit that AMs are a key component of the gut-lung axis, wherein an individual’s microbiota in its entirety is a determinant of disease outcome following exposure to an array of respiratory viruses.”
As the scientific community continues to unravel the complexities of the gut-lung axis, these findings pave the way for innovative approaches in manipulating the gut microbiota to enhance immune defenses against respiratory viral infections, potentially revolutionizing our strategies for preventing and treating these diseases in the future.

Leave a Reply